| Code | Description | Length |
| TCS001 | LaproClose – Introducer and 10/12mm guide | 15cm |
| TCS002 | LaproClose – Introducer only | 15cm |
| TCS003 | LaproClose – Introducer and 15mm guide | 15cm |
Trocar Site Closure Device (TSC001, TSC003)
Description
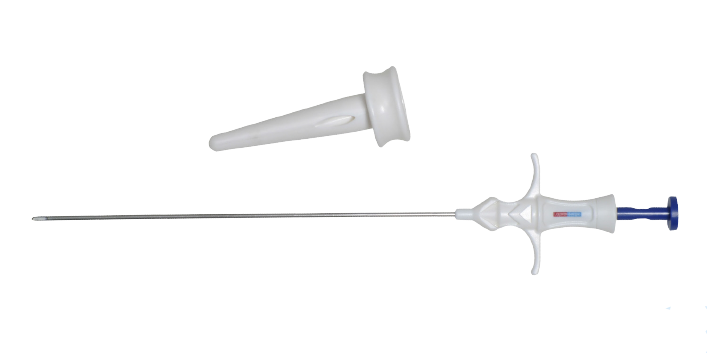
The LaproClose Trocar Site Closure device is a sterile and single-use product. It incorporates a 10mm/12mm/15mm cone shaped guide, and an introducer with retractable jaws. The device has application in laparoscopic procedures for approximation of tissues and percutaneous suturing for closing incision sites. The device must be used with a suture of appropriate size to close the incision site.
Contraindications
1. Do not use where laparoscopic techniques are generally contraindicated.
2. Do not use the trocar site closure device where is not possible to visualize the tip of the device during insertion or removal.
Instruction for Use
1. Insert the guide with the holes aligned vertical to the trocar wound. Depress the top button to expose the jaws inside the introducer and load the suture. Puncture the needle through the guide, fascia, muscle and peritoneum into the abdomen. Drop the suture and remove the introducer.
2. Insert the introducer through the other hole of the guide. Puncture the needle through all the layers and pick up the suture with the jaws of the introducer.
3. Pull the suture up through the peritoneum, muscle, fascia and guide. If using the 15mm guide, position the introducer with hole 1 and 2 aligned vertical to the trocar wound (please refer to the image below). Perform step 1-3 to hole 1 and 2, and repeat step 1-3 again to hole 3 and 4.
4. Remove the guide and tie off the suture.
Warnings and Precautions
1. This device is designed for single use only, and is not to be reused or re-sterilised. Please do not re-use or re-process to avoid irreversible injury such as cross-infection or injury caused by device malfunction. Do not Use if package is opened or damaged.
2. Endoscopic procedures should be performed only by physicians with adequate training and knowledge of these procedures. In addition, medical literature should be consulted regarding techniques, hazards, contraindications, and complications prior to the performance of these procedures.
3. Before endoscopic instruments and accessories from different manufacturers are utilised together in a procedure, verify compatibility and ensure that electrical isolation of ground is not compromised.
4. Store this device in a dry, clean and safe place. Keep the device away from flammable and combustible chemicals or materials.
5. Please follow universal guidelines upon medical waste disposal to avoid any potential cross-infection, environmental contamination or injury caused by sharp waste.
• Do not depress the button during insertion of LaproClose trocar site closure device; to do so may prevent the needle tip from penetrating tissue.
• Do not depress the button during removal of LaproClose trocar site closure device to ensure that the hooked end does not inadvertently catch tissue.
• Always visualize the tip either directly or through a laparoscope during insertion and removal of LaproClose trocar site closure device to prevent an unintentional puncture of underlying organs.
• After removing LaproClose trocar site closure device from the abdominal cavity, always inspect the site for hemostasis.
Trocar Site Closure Device (TSC002)
Description
The LaproClose Trocar Site Closure device is a sterile and single-use product, incorporating an introducer with retractable jaws. The device has application in laparoscopic procedures for approximation of tissues and percutaneous suturing for closing incision sites. The device must be used with a suture of appropriate size to close the incision site.
Contraindications
1. Do not use where laparoscopic techniques are generally contraindicated.
2. Do not use the trocar site closure device where is not possible to visualize the tip of the device during insertion or removal.
Instruction for Use
1. To expose the jaws inside the stylet, depress and hold down the top button.
2. Once the jaws are exposed, grasp the suture with the jaws. Release the button to catch the suture with the cannula.
3. Insert the suturing device through one side of the incision, just below the level of the skin, incorporation the fascia. Caution: Ensure that the button is not depressed during insertion.
Caution: Always visualise the tip either directly or through a laparoscope during insertion and removal of LaproClose trocar site closure needle to prevent an unintentional puncture of underlying organs.
4. Upon visualisation of the tip, either directly or through a laparoscope, depress the button to release the suture.
5. Release the button and withdraw LaproClose trocar site closure needle. Tag the suture ends with a clamp before reinserting LaproClose trocar site closure needle. This will prevent the suture from being pulled prematurely into the body cavity.
6. Reinsert LaproClose trocar site closure needle (without suture) on the opposite side of the incision, incorporating the fascia. Upon entry, visualise the tip of LaproClose trocar site closure needle.
7. While depressing the button to expose the jaws of the stylet, grasp the suture. Once the suture is grasped, release the button and remove LaproClose trocar site closure needle.
8. Proceed with a standard knot tying technique to close the incision site.
9. If additional stitches are required, repeat steps 1 through 8.
Warnings and Precautions
1. This device is designed for single use only, and is not to be reused or re-sterilised. Please do not re-use or re-process to avoid irreversible injury such as crossinfection or injury caused by device malfunction. Do not Use if package is opened or damaged.
2. Endoscopic procedures should be performed only by physicians with adequate training and knowledge of these procedures. In addition, medical literature should be consulted regarding techniques, hazards, contraindications, and complications prior to the performance of these procedures.
3. Before endoscopic instruments and accessories from different manufacturers are utilised together in a procedure, verify compatibility and ensure that electrical isolation of ground is not compromised.
4. Store this device in a dry, clean and safe place. Keep the device away from flammable and combustible chemicals or materials.
5. Please follow universal guidelines upon medical waste disposal to avoid any potential cross-infection, environmental contamination or injury caused by sharp waste.
• Do not depress the button during insertion of the suturing device; to do so may prevent the needle tip from penetrating tissue.
• Do not depress the button during removal of the suturing device to ensure that the hooked end does not inadvertently catch tissue.
• Always visualize the tip either directly or through a laparoscope during insertion and removal of the suturing device to prevent an unintentional puncture of underlying organs.
• After removing the suturing device from the abdominal cavity, always inspect the site for hemostasis
LaproClose Trocar Site Closure
Complete system for quick, safe and reliable closure of laparoscopic incision sites.
DESCRIPTION
Cone-shaped guide for closure of 10mm/12mm incisions:
- Correctly angles the introducer to capture all tissue layers for full closure of fascia and peritoneum
- Prevents loss of pneumoperitoneum during port site closure
Rigid introducer for passing sutures through all tissue layers:
- Needle guides easily through fascia and peritoneum.
- Ergonomic handle for comfortable finger placemen.
- Long, retractable grasping jaws to accommodate any suture size.
- Intuitive spring mechanism for extending and retracting the grasping jaws.
The combined use of the guide and introducer together minimises the risk of internal injuries by ensuring the introducer passes through the abdominal wall in an acute angle, enabling better visualisation of the needle entering the peritoneal cavity.